What has happened?
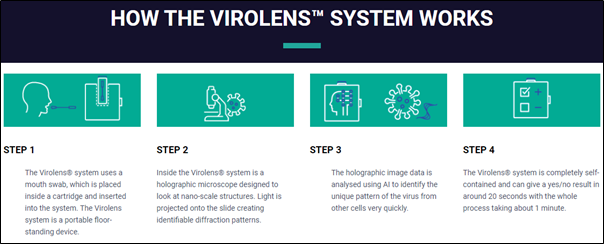
XTEK (ASX:XTE) today announced that they have signed an exclusive distribution agreement with KeyOptions Pty Ltd for the sale and support of Virolens® in Australia, New Zealand and the Pacific independent states. Virolens® is a recently developed technology that can screen for SARS-CoV-2 in approximately 20 – 30 seconds and has been accepted by the UK Medical and Healthcare product Regulatory Agency (MHRA) with an application having been lodged by KeyOptions with the Therapeutic Goods Administration (TGA) in Australia as well.
What are the key highlights?
- The Company reported that it has signed an exclusive distribution agreement with KeyOptions for the sale, distribution and support of Virolens®, a rapid COVID-19 diagnostic technology.
- The signed agreement between XTEK and KeyOptions is set to initially last for two years, with year-to-year automatic renewal thereafter. Exclusivity of the contract is subject to minimum sales requirements in year 1.
- Virolens® can screen for COVID-19 in approximately 20-30 seconds. The proof of concept demonstrated the Virolens® system had a 98.1% sensitivity and 99.7% specificity, based on the results of an internal in-vitro validation study of 184 samples.
- The Virolens® product has been accepted by the UK MHRA for registration as an in vitro diagnostic (IVD) Medical Devices Directive 98/79/EC; and an application has been lodged with the TGA for registration as a Class 1 IVD medical device.